Healthcare, pharma, and medtech companies operate in one of the most complex and rapidly evolving markets. As AI-driven diagnostics, digital therapeutics, and precision medicine advance, the pressure to launch effectively—and remain compliant—has never been greater. A robust Go-to-Market (GTM) strategy can be the deciding factor between stagnation and sustainable growth, particularly given new considerations such as real-world evidence (RWE) requirements, evolving FDA guidelines for AI-based devices, and Europe’s ongoing transition to MDR (Medical Device Regulation).

Key Takeaways
-
Regulatory Strategy Is Essential
Plan for evolving global regulations (e.g., EU MDR, FDA’s AI guidance) and privacy laws. Early compliance fosters credibility and market confidence. -
AI Unlocks Targeted Opportunities
Harness AI-driven analytics to identify niche patient groups, forecast disease prevalence, and refine messaging based on specific clinical needs. -
Automation Enhances Efficiency and Visibility
CRM and marketing automation tools streamline sales pipelines, deliver personalized content, and provide real-time performance tracking. -
Omnichannel is Non-Negotiable
Modern HCPs and patients interact across digital and live channels; an integrated approach ensures consistent messaging and better engagement data. -
Value-Based Narratives Drive Adoption
Pair your product’s clinical benefits with financial ROI to resonate with increasingly budget-conscious healthcare systems and payers. -
Thought Leadership Builds Authority
Producing expert content—from research-backed white papers to interactive webinars—positions you as a trusted advisor, accelerating market adoption. -
Measure, Optimize, Repeat
Use real-world evidence and analytics to continuously refine market segmentation, messaging, and overall GTM strategy.
Key Challenges in Healthcare GTM
-
Regulatory Complexity
- Global Regulatory Bodies: Companies must navigate frameworks from the FDA (U.S.) to the EMA (Europe) and beyond. The extended transition to Europe’s MDR and IVDR regulations adds layers of uncertainty and compliance demands.
- Data Privacy and Security: Beyond HIPAA and GDPR, new data-sharing requirements for real-world evidence make compliance even more intricate.
- Highly Informed Stakeholders
-
Elevated Expectations: Physicians, payers, and patients access real-time medical research and health tech updates. They demand robust clinical evidence and transparent value propositions.
-
New Decision-Making Influencer: The rise of patient advocacy groups and online health communities means patients are playing a more active role in treatment decisions.
-
-
Fragmented Markets
-
Localized Dynamics: Healthcare systems and reimbursement models differ widely across regions. Tailoring GTM strategies—from messaging to pricing—remains crucial.
-
Varying Payer Mixes: The ratio of public vs. private payers can drastically affect coverage decisions and how you position product value.
-
-
Innovation Overload
-
-
Rapid Technological Shifts: AI, IoT, and digital therapeutics offer vast opportunities but also demand swift and effective market education.
-
Accelerated Regulatory Oversight: The FDA’s evolving framework for Software as a Medical Device (SaMD) underscores the need for agility and compliance as innovations emerge.
-
GTM Innovations Driving Success
Below are key GTM methodologies and tools revolutionizing how healthcare, pharma, and medtech organizations bring new solutions to market.
1. AI-Powered Market Segmentation
AI models can integrate real-world data, patient registries, and demographic trends to uncover hidden market segments and predict disease outbreaks. Benefits include:
-
Precision Targeting: Identify subpopulations most likely to benefit from your product—whether by age, condition, or geography.
-
Predictive Demand Analysis: Forecast areas of high disease prevalence to guide resource allocation and local marketing.
-
Tailored HCP Outreach: Personalize messaging for specialists who require detailed clinical insights and ROI data.
2. CRM and Marketing Automation Tools
Software like Salesforce, Epic (Cheers), Veeva Vault (Life Sciences), Marketo, and HubSpot remain go-to solutions for streamlining lead management, nurturing, and analytics:
-
Seamless Lead Nurturing: Customize drip campaigns based on specific HCP interests (e.g., cardiology, oncology).
-
Real-Time Pipeline Visibility: Monitor sales progress, performance metrics, and forecast accuracy on intuitive dashboards.
-
Scalable AI Insights: Utilize automated lead scoring to focus sales efforts where you have the highest closure probability.
3. Omnichannel Communication Strategies
Modern stakeholders—physicians, administrators, patients, and even investors—are active on multiple platforms:
-
Consistent Brand Voice: Ensure every touchpoint, from LinkedIn posts to conference presentations, aligns with your messaging.
-
Enhanced Engagement: Engage HCPs and patients where they spend time, whether that’s webinars, social media, or live events.
-
Holistic Data Tracking: Track each interaction to measure marketing effectiveness and channel ROI.
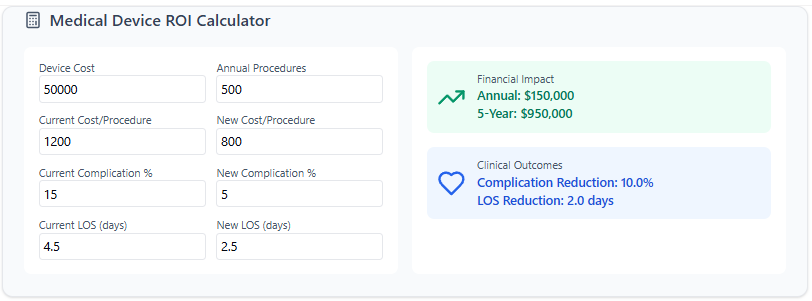
4. Value-Based Selling with ROI Calculators
As healthcare shifts to value-based care, decision-makers increasingly demand evidence of clinical and financial benefits:
-
Quantifiable Cost Savings: Demonstrate reduced readmissions, faster recoveries, or improved quality of life.
-
Informed Budget Decisions: Procurement teams and hospital administrators can see exact ROI estimations, simplifying purchase decisions.
-
Evidence of Real-World Efficacy: Align ROI calculators with real-world data to reinforce credibility in outcomes-based contracts.
5. Thought Leadership and Content Marketing
Establishing credibility is critical in a sector where trust is paramount. Engaging, authoritative content can include:
-
White Papers & Webinars: Deep dives on emerging trends like AI in diagnostics or molecular-level targeted therapies.
-
Case Studies with Real-World Evidence: Showcase successful implementations, with details on improved patient outcomes and reduced total cost of care.
-
Expert Interviews & Panels: Collaborate with Key Opinion Leaders (KOLs) to amplify your voice and extend reach.

GTM Innovations Driving Success
Illustrative Example – Mecuris and 3D-Printed Prosthetics
Mecuris exemplifies how targeted GTM can revolutionize patient care in medtech. Its digital platform—Mecuris Solution Platform (MSP)—enables the customization and 3D printing of prosthetic and orthotic devices. Highlights include:
- Market Analysis
- Thorough research in initial markets (e.g., Germany, Spain) to gauge demand and validate product fit.
- Direct feedback from orthopedic professionals, payers, and end-users informed both product features and messaging.
- Product Positioning
- Emphasis on German engineering and stringent quality control to appeal to providers and payers seeking reliable, high-tech solutions.
- Differentiation built on precision, customization, and cost-effectiveness.
- Regulatory Navigation
- Compliance with EU’s CE marking requirements showcased Mecuris’s commitment to patient safety and product efficacy.
- Early engagement with regulatory bodies helped streamline market entry and minimize risks.
- Customer Engagement
- Hands-on demos, workshops, and real-time Q&A sessions accelerated product adoption among orthopedic professionals.
- Iterative feedback loops made the MSP more user-friendly, strengthening its competitive edge.
- Strategic Prioritization
- Focus on high-impact product lines (e.g., prosthetic “Covers”) led to successful rollouts in targeted geographies.
- Partnering with distributors and KOLs scaled brand awareness and market penetration.
Best Practices for GTM in Healthcare
-
Invest in Data Analytics
-
Advanced analytics drive product positioning, regional forecasts, and even R&D priorities.
-
Real-world evidence (RWE) is increasingly central to both regulatory approvals and payer acceptance.
-
-
Prioritize Compliance from Day One
-
Factor in FDA guidelines, HIPAA/GDPR, and the evolving EU MDR/IVDR. Compliance not only mitigates risk but also builds stakeholder trust.
-
Consider specialized consultants or in-house teams focused solely on regulatory updates.
-
-
Focus on Customer-Centricity
-
Understand the unique pain points of each stakeholder—HCPs, payers, patients.
-
Engage KOLs early to validate your messaging and refine product benefits.
-
-
Leverage Strategic Partnerships
-
Collaborations with academic institutions and hospital networks can accelerate clinical validation and market acceptance.
-
Industry consortia offer platforms for shared learnings and co-development opportunities.
-
-
Measure and Optimize
-
Implement robust KPIs: monitor adoption rates, clinical outcomes, and user feedback to continually refine your GTM approach.
-
Use analytics dashboards to track metrics in real time and make agile adjustments.
-
Conclusion
A successful GTM strategy in the healthcare, pharma, and medtech sectors demands more than a conventional sales playbook. It requires a nuanced approach that aligns with evolving regulations, leverages breakthrough technologies like AI and real-world evidence, and speaks directly to well-informed stakeholders.
By integrating AI-powered segmentation, marketing automation, omnichannel communication, ROI-based selling, and thought leadership, healthcare innovators can navigate an increasingly complex ecosystem while advancing patient care. Whether you’re a nascent startup or a global enterprise, these GTM tools and tactics will help you stand out, drive sustainable growth, and—most importantly—improve patient outcomes.

