Date: February 5, 2026 | Sector Lens: Medical Imaging + AI (Breast screening / mammography workflow)
ScreenPoint Medical’s Transpara-enabled MASAI randomized trial published its primary endpoint in The Lancet, showing a non-inferior interval cancer rate versus standard double reading with higher sensitivity and unchanged specificity. We interpret this as a market signal that breast AI is shifting from a productivity tool to outcomes-linked screening infrastructure, raising the evidence and governance bar for vendors and buyers.
ScreenPoint Medical’s Transpara-enabled MASAI randomized trial published its final endpoint analysis in The Lancet, showing non-inferior interval cancer rates for AI-supported screening versus standard double reading, with higher sensitivity and similar specificity.
The Market Signal: Breast AI has graduated from a ‘productivity add-on’ to ‘procurement-grade infrastructure.’ With MASAI, buyers now have the outcomes-linked evidence required to rewrite tender scoring and hard-code AI into staffing models.
Next watch: generalizability, governance, and contracting—multi-device, multi-population evidence; real-world drift monitoring; and how programs translate interval-cancer deltas into reimbursement/standards.
Key Takeaways
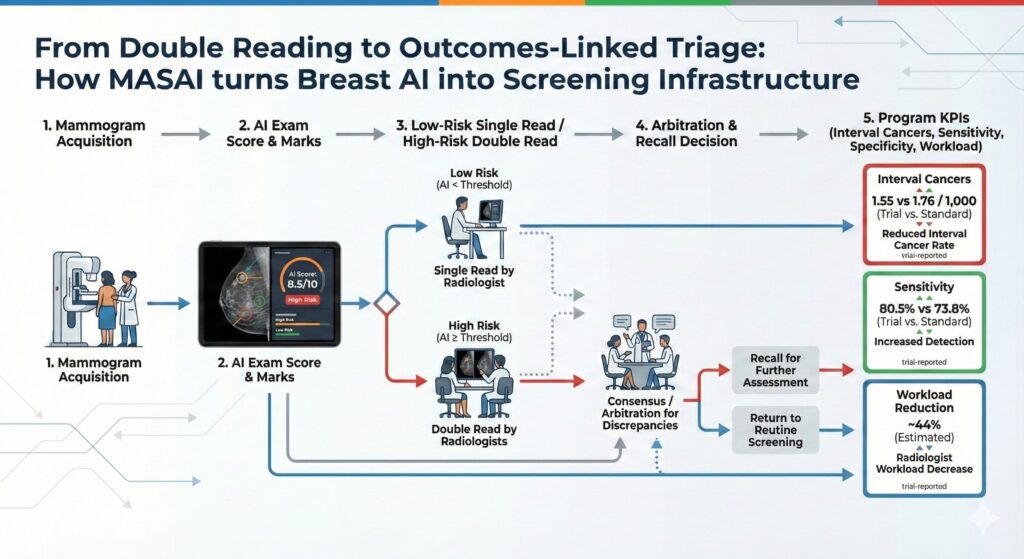
- Signal: MASAI’s primary endpoint readout makes breast screening AI “decision-grade” for policy and procurement: interval cancer rate met non-inferiority with directional improvement.
- Value capture: The economic story is capacity + quality simultaneously: earlier MASAI analyses show ~44% lower screen-reading workload, while later analyses show higher cancer detection without higher false positives.
- Who benefits: European programs facing double-reading constraints gain the cleanest ROI; U.S. providers gain decision-support value but will need a different workflow justification (since routine double reading is uncommon).
- Competitive implication: This evidence accelerates the shift from point tools to “embedded workflow operating models” (triage + QA + detection + governance) and will pressure competitors to match outcomes evidence, not just AUC.
- Outlook: Expect 2026–2027 tenders to explicitly score vendors on outcomes endpoints + auditability + implementation maturity—and to favor vendors with cloud-scalable distribution and fleet-level integration.
The Event
- What happened: The Lancet published final results from MASAI (Mammography Screening with Artificial Intelligence), a Swedish randomized, controlled, non-inferiority, single-blinded, population-based screening accuracy trial comparing AI-supported screening vs standard double reading without AI.
- When: Publication date Jan 29, 2026; ScreenPoint Medical highlighted the publication on Jan 30, 2026.
- Scale: 105,934 women randomized (April 2021–Dec 2022), with 19 excluded from analysis.
- Primary endpoint: Interval cancer rate (non-inferiority margin 20% per protocol).
- Topline results (primary endpoint + key secondary measures):
- Interval cancer rate: 1.55 vs 1.76 per 1,000 (AI-supported vs control), proportion ratio 0.88, meeting non-inferiority (p=0.41; trial not powered for superiority).
- Sensitivity: 80.5% vs 73.8% (p=0.031).
- Specificity: 98.5% vs 98.5% (p=0.88).
- Interval cancer characteristics (descriptive): fewer invasive interval cancers (75 vs 89), fewer T2+ (38 vs 48), fewer non-luminal A (43 vs 59) in the AI arm.
- Workflow model: AI used for triage to single vs double reading and as detection support highlighting suspicious findings.
Disclosure on terms: No deal terms; not a financing announcement. Commercial terms of Transpara deployments are not disclosed in the trial publication.
Exhibit: Event Snapshot (facts)
| Field | Detail |
| Event | MASAI final endpoint publication (The Lancet) |
| Publication date | Jan 29, 2026 (EurekAlert!) |
| Company relevance | AI workflow includes Transpara (ScreenPoint Medical) (PubMed) |
| Trial type | Randomized, controlled, non-inferiority, single-blinded, population-based screening accuracy trial (PubMed) |
| Participants | 105,934 randomized; 19 excluded (PubMed) |
| Primary endpoint | Interval cancer rate; 20% non-inferiority margin (PubMed) |
| Primary outcome | 1.55 vs 1.76 per 1,000; proportion ratio 0.88; p=0.41 (non-inferior) (PubMed) |
| Key secondaries | Sensitivity 80.5% vs 73.8% (p=0.031); specificity 98.5% both (p=0.88) (PubMed) |
Sources:
The Lancet MASAI trial publication (Jan 2026); Lancet Oncology MASAI safety analysis (Aug 2023); Lancet Digital Health MASAI screening performance analysis (2025); DeepHealth ASSURE study results (Nature Health, Nov 2025); FDA 510(k) indications for use for Transpara; select company disclosures and reputable trade reporting.
About Marketstrat
Marketstrat® is a market intelligence and GTM enablement firm focused on medtech, healthcare, and life sciences. Under the Markintel™ brand, Marketstrat delivers robust market intelligence and proprietary frameworks; through GrowthEngine advisory and tools, it helps clients turn insights into execution. Together, these capabilities support clients in converting evidence‑weighted insight into practical action across strategy, product, and commercial execution.
Marketstrat® and Markintel™ are trademarks of Marketstrat Inc. All other trademarks are the property of their respective owners.
Check out free Research and Insights and Analysis of Industry Events
Check out our collection of Markintel Horizon and Markintel Pulse research.
Check out details on our reports, World Market for AI in Medical Imaging and World Market for Oncology Imaging AI and other Pulse Reports in the Imaging space.

